Analysis of Nitrosamines in Sartan-Type Bulk Drug Substances
- In julio 2018, the American Food and Drug Administration (FDA) announced that the carcinogenic impurities: Nitrosamines (N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA)) had been detected in Valsartan bulk drug substances manufactured by Chinese manufacturers. Subsequently, a worldwide recall was issued of pharmaceutical products that use Valsartan bulk drug substances. Valsartan is used in the treatment of high blood pressure and congestive heart failure.
- The FDA has announced analysis methods using gas chromatography mass spectrometry (GC-MS and GC-MS/MS) as detection methods for NDMA and NDEA, while the European Directorate for the Quality of Medicines (EDQM) has announced analysis methods using liquid chromatography mass spectrometry (LC-MS/MS) and GC/MS as reference information.
- This article introduces an example of the analysis of NDMA and NDEA in Valsartan bulk drug substances utilizing a headspace sampler (HS-20) with GC-MS (GCMS-QP2020 NX) & GC-MS/MS (GCMS-TQ8040 NX) gas chromatograph mass spectrometers.
Analysis of NDMA and NDEA via Headspace GC-MS
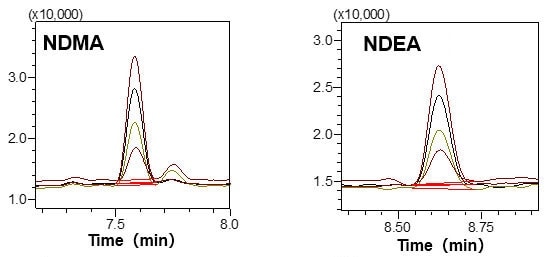
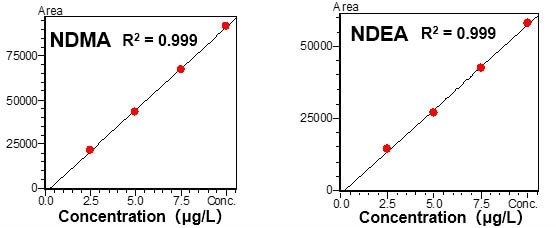
Standard solutions (DMSO solutions) prepared within the range of 2.5 to 10 μg/L of NDMA and NDEA, respectively, were measured with the HS-20 and the GCMS-QP2020 NX, to create chromatograms and calibration curves.
Favorable linearity was obtained with a contribution ratio (R2) of 0.999 for both components, well within the above-mentioned concentration range.
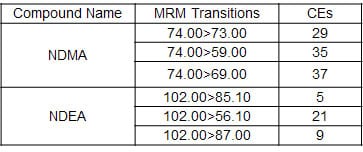
Concurrent Accuracy with the Headspace GC-MS
Utilizing the HS-20 and the GCMS-QP2020 NX, standard solutions (DMSO solutions) prepared with 5.0 μg/L of NDMA and NDEA, respectively, were injected repeatedly over six cycles, and the peak area values were calculated. Favorable repeatability was obtained even in the low concentration region of 5.0 μg/L.
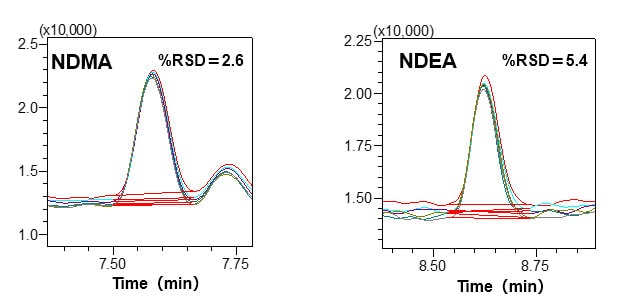
Repeatability
Valsartan bulk drug substances, Losartan bulk drug substances, and Olmesartan bulk drug substances were dissolved in DMSO at 5 % (w/v). Samples with NDMA and NDEA added to ensure final concentrations of 2.5, 5.0, and 10 μg/L were then subjected to additive recovery tests utilizing the HS-20 and the GCMS-QP2020 NX. Favorable results were obtained, with recovery rates in the range of 88 to 114 %.
| API | Valsartan | Losartan | Olmesartan Medoxomil | |||
|---|---|---|---|---|---|---|
| Con c. | Recovery Rate (%) | Recovery Rate (%) | Recovery Rate (%) | |||
| NDMA | NDEA | NDMA | NDEA | NDMA | NDEA | |
| 2.5 μg/L | 108.6 | 104.6 | 95.0 | 113.4 | 103.4 | 114 |
| 5.0 μg/L | 99.9 | 96.5 | 96.1 | 107.9 | 89.8 | 88.7 |
| 10.0 μg/L | 100.5 | 114.2 | 99.8 | 107.7 | 98.3 | 102.7 |
Conditions
| Headspace gas sampler | :HS-20 |
| Oven temperature | :120 °C |
| Sample line temperature | :125 °C |
| Transfer line temperature | :130 °C |
| Gas pressure for vial compression | :103 kPa |
| Vial warming time | :15 minutes |
| Vial compression time | :1 minute |
| Compression equilibration time | :0.10 minutes |
| Injection time | :1 minute |
| Needle flush time | :1 minute |
| Sample injection volume | :1 mL |
| GC Column: | :SH-PolarWax (30 m x 0.25 mm x I.D,0.50 μm) |
| Carrier gas | :He |
| Control mode | :Pressure |
| Oven temperature |
:40 °C(2 minutes)→10 °C/minute
→120 °C→ 25 °C/minute → 230°C(5-6 minutes)
|
| Solvent |
N, N-Dimethyl sulfoxide
|
| Instrument | GCMS-QP2020 NX with HS-20 |
| MS (EI Method) Ion source temperature | :200 °C |
| Interface temperature | :230 °C |
| Tuning mode | :Standard |
| Measurement mode |
:NDMA SIM(m/z 74.0)
:NDEA SIM (m/z 102)
|
| Event time | :0.30 seconds |
Analysis of NMDA and NDEA via Headspace GC-MS/MS
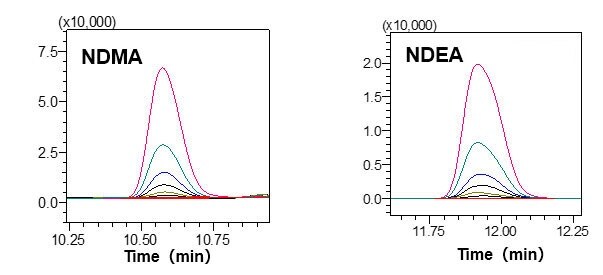
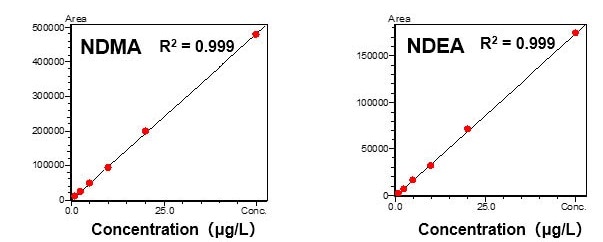
Standard solutions (DMSO solutions) prepared with 1.0 to 50 μg/L of NDMA and NDEA, respectively, were measured with the HS-20 and the GCMS-TQ8040 NX, to create chromatograms and calibration curves.
Favorable linearity was obtained with a contribution ratio (R2) of 0.999 for both components within the above-mentioned concentration range.
Concurrent Accuracy with the Headspace GC-MS/MS
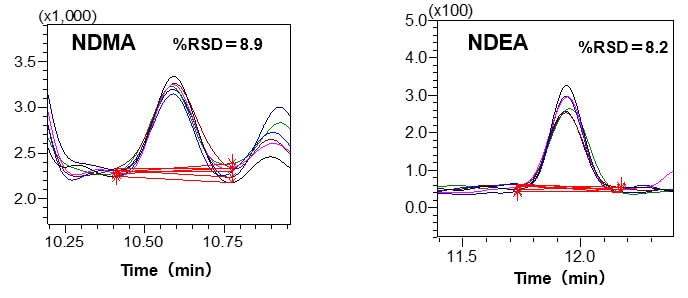
Utilizing the HS-20 and the GCMS-TQ8040 NX, standard solutions (DMSO solutions) prepared with 1.0 μg/L of NDMA and NDEA, respectively, were injected repeatedly over six cycles, and the peak area values were calculated.
Favorable repeatability results lower than 10 % were obtained, even in the low concentration region of 1.0 μg/L.
Repeatability
Valsartan bulk drug substances, Losartan bulk drug substances, and Olmesartan bulk drug substances were dissolved in DMSO at 2 % (w/v). Samples with NDMA and NDEA added to ensure final concentrations of 2.5, 5.0, and 10 μg/L were then subjected to additive recovery tests utilizing the HS-20 and the GCMS-TQ8040 NX. Favorable results were obtained, with recovery rates in the range of 83 to 119 %.
| API | Valsartan | Losartan | Olmesartan Medoxomil | |||
|---|---|---|---|---|---|---|
| Con c. | Recovery Rate (%) | Recovery Rate (%) | Recovery Rate (%) | |||
| NDMA | NDEA | NDMA | NDEA | NDMA | NDEA | |
| 2.5 μg/L | 109.5 | 858 | 101.2 | 83.0 | 113.3 | 119.1 |
| 5.0 μg/L | 97.4 | 100.5 | 101.9 | 99.1 | 114.2 | 96.1 |
| 10.0 μg/L | 103.7 | 105.9 | 106.4 | 110.4 | 119.4 | 100.9 |
Conditions
| Headspace gas sampler | :HS-20 |
| Oven temperature | :160 °C |
| Sample line temperature | :165 °C |
| Transfer line temperature | :175 °C |
| Gas pressure for vial compression | :80 kPa |
| Vial warming time | :25 minutes |
| Vial compression time | :1 minute |
| Compression equilibration time | :1 minutes |
| Injection time | :1 minute |
| Needle flush time | :1 minute |
| Sample injection volume | :1 mL |
| GC Column: | :SH-PolarWax (30 m x 0.25 mm x I.D,0.50 μm) |
| Carrier gas | :He |
| Oven temperature |
:40 °C(0 minutes)→8 °C/minute
→140 °C→ 25 °C/minute → 230°C(3-9 minutes)
|
| Solvent |
N, N-Dimethyl sulfoxide
|
| Instrument | GCMS-TQ8040 NX with HS-20 |
| MS (EI Method) Ion source temperature | :200 °C |
| Interface temperature | :230 °C |
| Tuning mode | :Standard |
|
|
|
![]()

GCMS-QP2020NX
A GC-MS is a general-purpose analysis instrument used in a variety of fields. The GCMS-QP2020NX heightens efficiency and can be expected to contribute to improved cost performance and a better work/life balance for users. Featuring enhanced instrument functionality, analysis software, databases, and a sample introduction system, the GCMS-QP2020 will help maximize the capabilities of your laboratory.

GCMS-TQ8040NX
The Shimadzu GCMS-TQ8040 is the first triple quadrupole with Smart Productivity for high efficiency sample throughput, Smart Operation for quick and easy method development, and Smart Performance for low detection limits and Scan/MRM.
These 3 smart technologies contribute to Smart MRM, and provide the most accurate, cost effective, and easy-to-use triple quadrupole GCMS you have ever imagined

LabTotal Vial
- High Quality Silicon Polymer and Cleaning Technique Provides Low Blank Level
- Wide Mouth with Preset Cap - "Ready To Use"
- Included Quality Certificate Guarantees High MS Grade Quality