Analysis of Favipiravir in Human Plasma Using Fully Automated Sample Preparation LC/MS/MS System
Favipiravir (brand name: Avigan®), which was developed by FUJIFILM Toyama Chemical Co., Ltd, is one of the RNA polymerase inhibitors used for treating influenza. In Application News C229, we introduced a robust, highlysensitive analysis using LC/MS/MS with manual pretreatment. However, manual pretreatment of plasma samples entails a certain level of workload. This report introduces a method of analyzing favipiravir using a fully automated sample preparation LC/MS/MS system that can reduce variation between procedures, sample mix-ups, and risk of exposure to the samples (Fig. 1).
Fully Automated Sample Preparation of Favipiravir in Plasma
For analysis of low-molecular weight compounds in plasma using a LCMS™, it is common to use supernatant collected following deproteinization by adding an organic solvent. With the fully automated sample preparation LC/MS/MS system, these preparatory steps are done automatically just by placing a blood collection tube in the system after plasma separation (Fig. 2). Pretreatment of the next sample can also be performed in parallel with LC/MS/MS analysis, which can greatly reduce the time required to analyze each sample. This analysis was performed in a per-sample cycle time of 6.5 minutes from plasma pretreatment to the analysis of favipiravir using LC/MS/MS.
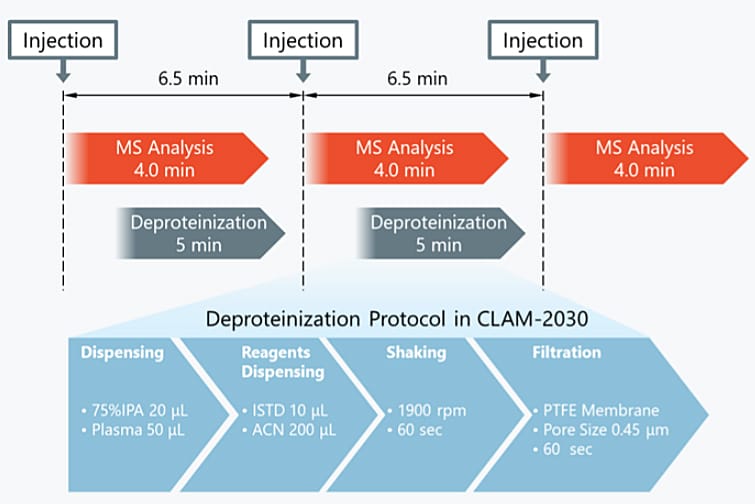
Fig. 2 Workflow of Fully Automated Sample Pretreatment
Analytical Conditions and Pretreatment of Samples
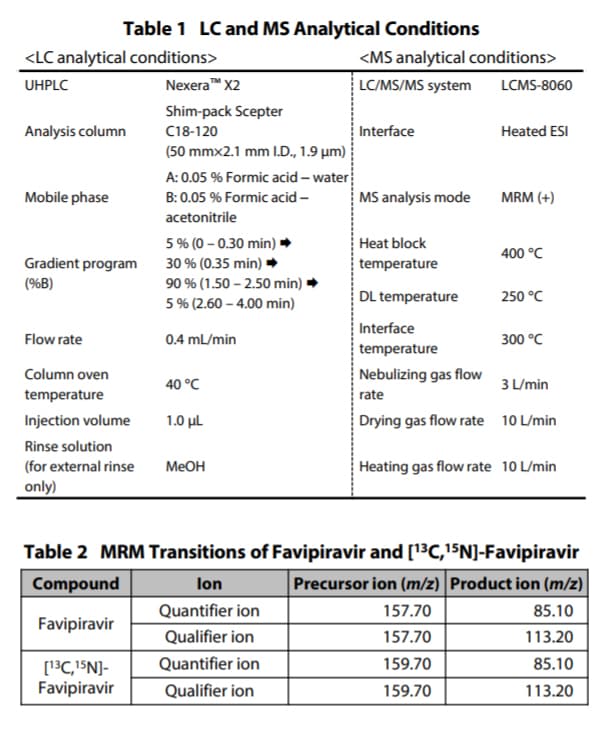
Favipiravir (PN: C8720*1), as the target compound, and[13C,15N]-favipiravir (PN: C8853*1), as its stable isotope, were purchased from Alsachim, one of the companies of the Shimadzu Group. [13C,15N]-favipiravir was used as the internal standard (ISTD). Favipiravir was spiked with commercially available human plasma treated with EDTA 2K to prepare calibration curves and QC samples. Analysis was performed using the LC and MS analytical conditions shown in Table 1 and the MRM transition in Table 2. Shimpack Scepter™ C18-120 (50 mm×2.1 mm I.D., 1.9 µm, P/N: 227-31012-03) was used as the analytical column. Fig. 3 shows the MS chromatograms and structural formulas of the compounds. A calibration curve was prepared using calibration points at plasma concentrations of 1, 2, 5, 10, 20, 50 and 100 µg/mL for favipiravir (n = 5 for each calibration point). [ 13C,15N]- favipiravir (20 µg/mL) solution was prepared using acetonitrile and used as ISTD. The pretreatment steps for the plasma sample spiked with favipiravir are shown in Fig. 2. Samples were automatically prepared through the following series of steps: mixing 20 μL of 75% isopropyl alcohol (IPA), 50 μL of plasma, 10 μL of ISTD and 200 μL of acetonitrile, shaking the mixture, and then filtration of the mixture using a PTFE membrane filter. Finally, the prepared sample was used for analysis. *1 Shimadzu GLC and Alsachim Product numbers
caption
![MS Chromatograms and Structural Formulas of Favipiravir (Left) and [13C,15N]-Favipiravir (Right)](http://www.shimadzu.com/an/sites/shimadzu.com.an/files/d7/ckeditor/an/industry/small_molecule_pharmaceutical/Preclinical/c230/f-3.jpg)
MS Chromatograms and Structural Formulas of Favipiravir (Left) and [13C,15N]-Favipiravir (Right)



