i-Series Method Transfer System

Enables Both HPLC and UHPLC Analysis
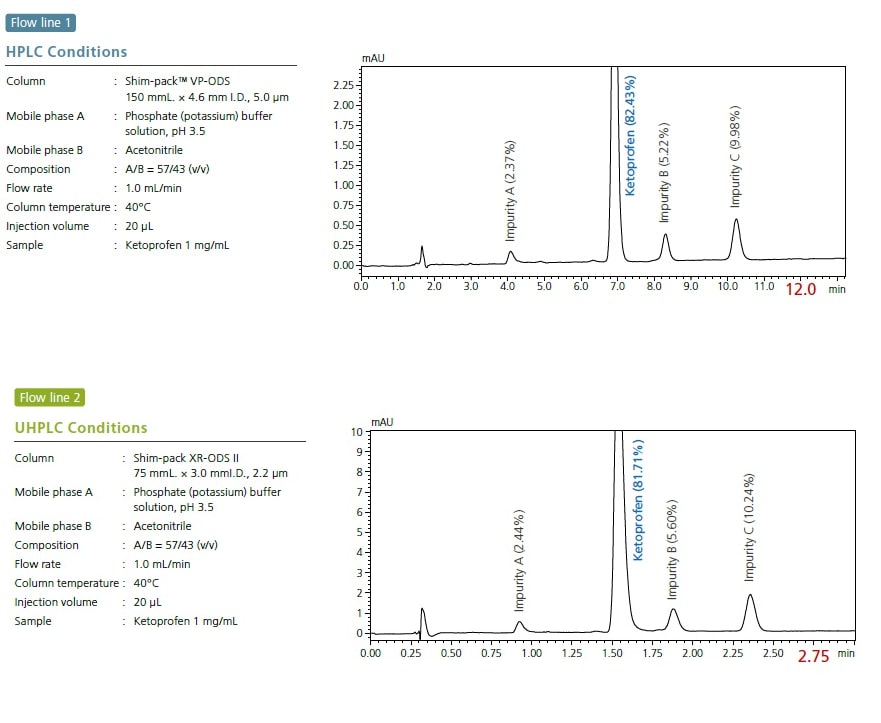
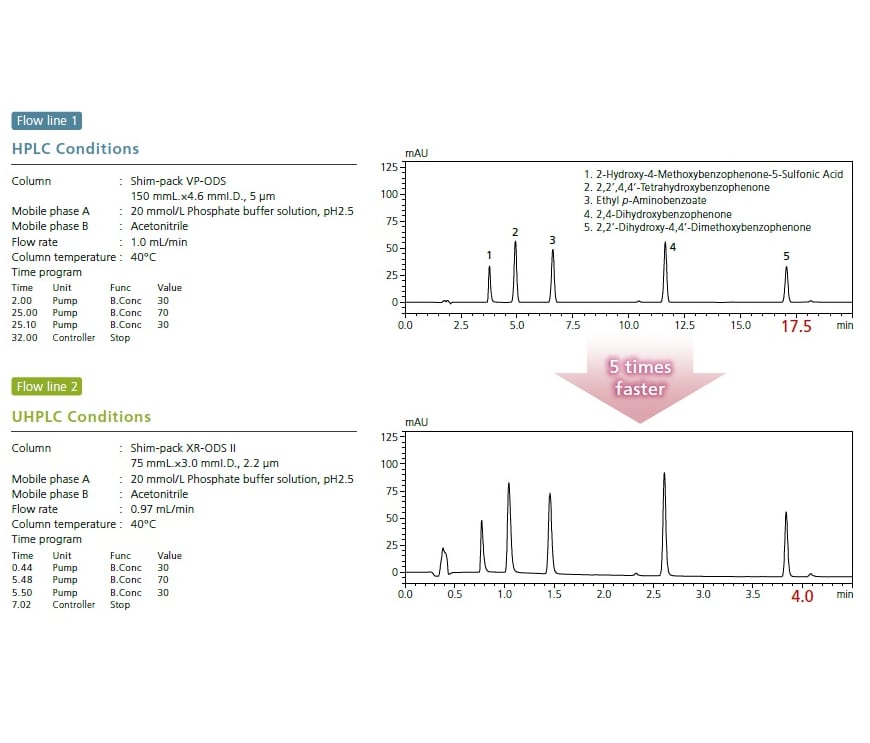
The i-Series Method Transfer System (MT system) enables both conventional analysis via its high compatibility with existing LC systems and rapid analysis via high-speed methods.In pharmaceutical development, a single integrated system allows process synthesis screening analysis for synthetic substances in the UHPLC flow line, and impurity content identification in the HPLC flow line.
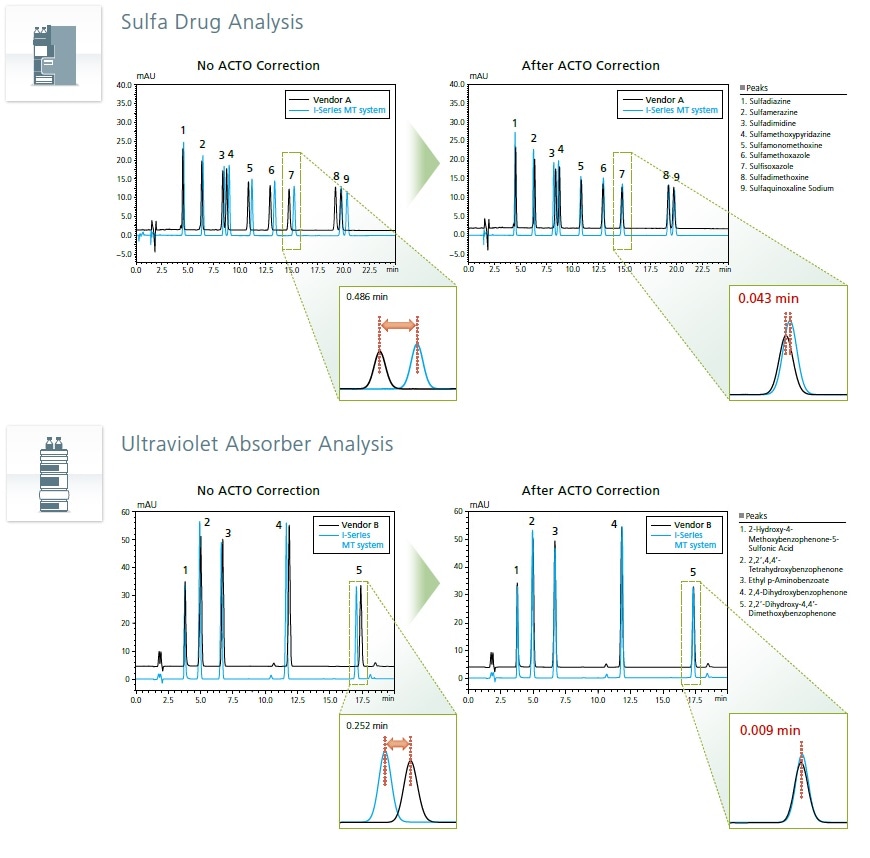
Analysis results from LC systems in the same laboratory may differ, even with the same method, due to differences in the system delay volumes of these systems. The i-Series MT system demonstrates outstanding reproducibility between various LC systems with different system delay volumes, simplifying method transfer between instruments.
Further more, with the i-Series MT system the process of migrating from UHPLC to HPLC or from HPLC to UHPLC can be accomplished using a single system.
The i-Series MT system is based on the same user-friendly i-Series, but maximizes performance and reliability for migrating and transferring customer methods.
Features
-
A single integrated system allows analyses in two flow lines with UHPLC and HPLC delay volumes. In addition to simplifying the transfer of analytical methods for customers using HPLC, it can also streamline the process of converting customers’ analytical methods from HPLC to higher-speed UHPLC.
-
The newly developed Analytical Condition Transfer and Optimization (ACTO) function incorporated in LabSolutions™ allows users to transfer injection timings matched to differences in system volumes between instruments, without editing the concentration gradient programs in existing methods.
-
Transfer Methods with a Single Button Operation—ACTO Function
-
Core Technologies That Provide a Solid Foundation for Analysis
Downloads
Download the latest brochure.
Technical Documents
| Documents | Date Creation Date |
|---|---|
2021-01-13 |
News / Events
-
New Method Development System
LabSolutions™ MD improves method development efficiency by taking an Analytical Quality by Design (AQbD) approach. This software efficiently develops highly reliable analysis methods by configuring mobile phases, columns, and other parameters using an analysis function that automatically generates analysis schedules with the experimental design method and a data analysis function that plots a design space and predicted chromatogram.
-
New Nexera Prep Preparative Purification Liquid Chromatograph
The Nexera™ Prep Purification System provides optimal solutions for your laboratory needs.
-
Shimadzu has released the Shim-vial™ H glass, S glass.
Shimadzu provides high-quality vials that thoroughly eliminate these risks by visually inspecting each vial, allowing them to be used with confidence.
-
Shimadzu has released the MUP-3100, Fully Automated Sample Preparation Module for Glycan Analysis
Shimadzu developed the MUP-3100 Fully Automated Sample Preparation Module for Glycan Analysis in conjunction with Sumitomo Bakelite Co., Ltd., for use with their antibody N-glycan analysis kit. The MUP-3100 eliminates manual sample preparation, increases overall analysis throughput, and improves reproducibility.
-
Ion Chromatography Solutions for Environmental Analysis
Shimadzu has focused on the development of instruments for environmental analysis for decades, helping scientists to detect, identify and quantify trace-level pollutants and meet environmental testing demands, for both regulatory purposes and to advance Research & Development.
-
Shimadzu has released the Peakintelligence™ for LC
Liquid chromatography is now an indispensable analytical technique used in pharmaceutical, food, and a wide variety of other industries. In these industries, there is a need for efficient data analysis methods that are not user-dependent. Peakintelligence for LC software includes AI algorithm* that was developed by learning expert peak integration skills.