The Horror of Sample Adsorption to Containers (Part 2)
Naoki Asakawa,
Technical Advisor, Analytical & Measuring Instruments Division,
Shimadzu Corporation
Part 1 described the mechanisms of sample adsorption to containers, and how to reduce this adsorption using commercialized low adsorption vials (Shim-vial). Recently, there have been a number of requests for the development of a low adsorption polypropylene (PP) pipette tip (PP tip). These requests come from users who have recognized absorption to standard PP tips, which are often used for sampling, dilution and other aspects of sample preparation, as a detriment to the reliability of analysis results.
Accordingly, continuing on from the TORAST-H Bio Vial, the Shimadzu Group began assessment of adsorption to PP tips, together with the development of a low adsorption PP tip, and in a world's first has now commercialized the TORAST-H Tip. In this section, we introduce the phenomenon of adsorption to PP tips, provide an overview of the TORAST-H Tip, and describe its adsorption-reducing effect.
Adsorption to PP Tips
Adsorption to PP tips consists mainly of hydrophobic adsorption. Accordingly, the most common adsorption-reducing method is to add an organic solvent (such as methanol or acetonitrile) to the sample solution (refer to Part 1). However, in accordance with the increase in speed of LC and LC/MS analysis in recent years, filler particle sizes and column sizes have become miniaturized. As a result, when sample solutions with a large amount of organic solvent are applied to LC columns in reverse phase mode, the peaks are broadened by the organic solvents in the samples and, in many cases, sufficient separation is not obtained.
In such cases, the response at present is to limit the sample injection volume or the amount of organic solvents added to the sample. Because of this, however, the inherent high-separation capacity and high-sensitivity detection of LC and LC/MS may not be sufficiently demonstrated. Accordingly, in order to maximize the performance of LC and LC/MS, organic solvent-free sample solutions would be preferable. The development of PP tips that suppress hydrophobic adsorption, of concern in such cases, is thus highly significant.
Development of the TORAST-H Tip
The Shimadzu Group has commercialized the TORAST-H Bio Vial low adsorption PP vial, with support from many of our users. In this tip, an ultra-hydrophilic/nonionic high molecular weight polymer is chemically bonded to the PP tip (untreated), suppressing hydrophobic adsorption on the surface of the PP tip (Fig. 1).
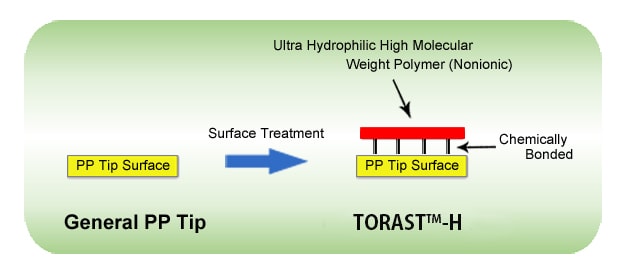
Fig. 1 Overview of the TORAST-H Tip
This chemical bonding is very stable, meaning that samples can be prepared with a wide range of solvents.
TORAST-H Tip Adsorption-Reducing Effect
In order to verify adsorption suppression by the TORAST-H Tip, the degree of adsorption was verified for this tip, an untreated tip, and a commercially available tip, using peptides and basic substances as the model samples.
1) Peptides
Peptides were introduced as subject because of their significant adsorption to containers (refer to Part 2). In this case, samples of trypsin digested myoglobin (≒1.9 pmol/mL) were placed in TORAST-H Bio Vials using the TORAST-H Tip, an untreated tip (the TORAST-H Tip base tip), and a commercially available tip, to form sample solutions. Fig. 2 shows the chromatograms for each sample solution, obtained by HPLC.
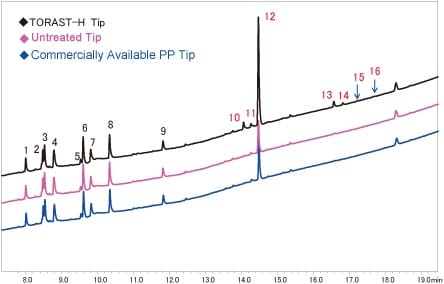
Column: Aeris Peptide (Internal diameter of 2.1 mm, length of 150 mm, particle size of 1.7 µm)
Mobile phase: 0.1 % TFA aqueous solution/0.1 % TFA acetonitrile solution, gradient elution
Detection: Ultraviolet 216 nm
At each peptide peak (No. 1 to 16), no differences in the peak areas for the hydrophilic peptides (No. 1 to 9 with small retention times) were evident as a function of the tip (Fig. 3).
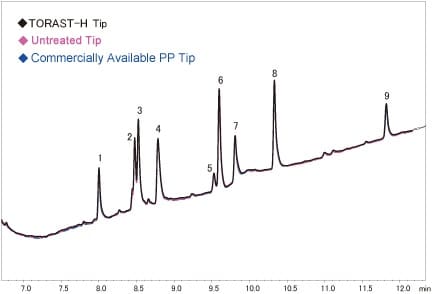
Fig. 3 Adsorption of Trypsin Digested Myoglobin to PP Tips
(Enlarged Chromatogram: Peak No. 1 to 9)
In contrast, regarding the peak areas for the hydrophobic peptides (No. 10 to 16 with large retention times), significant differences were evident as a function of the tip. With the untreated tip and the commercially available tip, the peak area was extremely reduced, by 60 % to 90 % in comparison to the TORAST-H Tip (Fig. 4). This suggests that adsorption to the PP tip is caused by hydrophobic adsorption. At the same time, it reveals that peptides are significantly adsorbed to PP tips, even at UV detectable sample concentrations. In contrast, the TORAST-H Tip showed significant adsorption suppression in comparison to the untreated tip and the commercially available tip.
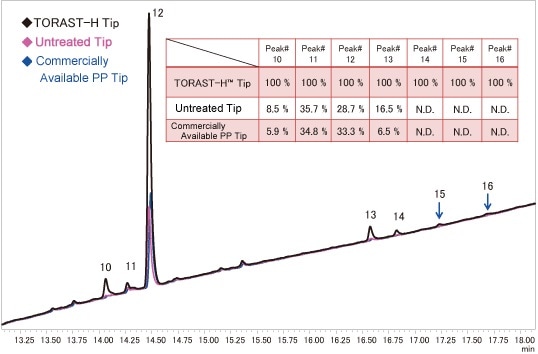
Fig. 4 Adsorption of Trypsin Digested Myoglobin to PP Tips
(Enlarged Chromatogram: Peak No. 10 to 16)
2) Basic Substances
Adsorption to the TORAST-H Tip and an untreated tip was compared by LC/MS (Nexera X2 and LCMS-8050) for low-concentration samples (1 ng/mL and 10 ng/mL aqueous solutions) using imipramine, a highly hydrophobic antidepressant, and atenolol, a highly hydrophilic beta blocker, as the model compounds.
i) Imipramine
Imipramine (logP: 4.28) is a highly hydrophobic medication for which adsorption to PP surfaces is a concern. In comparison to the TORAST-H Tip, the peak area for the untreated tip was decreased by 66 % at 10 ng/mL, and 74 % at 1 ng/mL. These results show that imipramine is easily adsorbed to the PP tip surface. Conversely, these results suggest that TORAST-H Tip has a significant adsorption-reducing effect (Fig. 5).
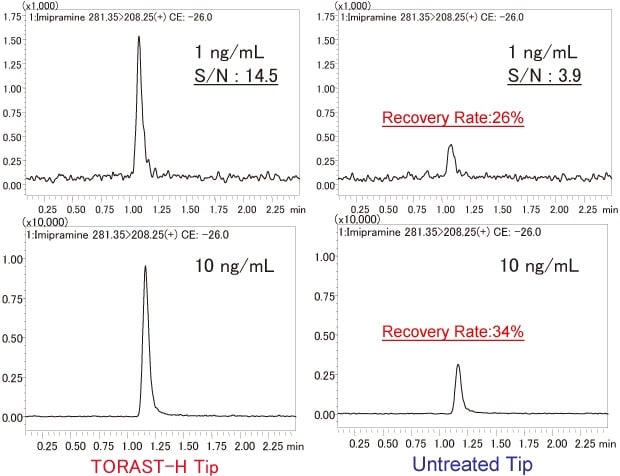
Fig. 5 Adsorption of Imipramine (1 ng/mL and 10 ng/mL) to PP Tips
(Chromatogram by LC/MS)
ii) Atenolol
Atenolol (logP: 0.43) is a highly hydrophilic medication. Low adsorption to PP tip surfaces is easily surmised. A 1 ng/mL aqueous solution of atenolol was sampled by a TORAST-H Tip and untreated tip, respectively, followed by transfer to TORAST-H Bio Vials to form sample solutions. The peak areas for these samples were compared using LC/MS. With the untreated tip, the peak area decreased slightly in comparison to the TORAST-H Tip. After this, as a model experiment in which a low-concentration sample was postulated, the area of contact with the tip was increased five and 10 times. The peak area with the untreated tip decreased 17 % and 30 %, respectively. These results suggest that even for highly hydrophilic compounds as typified by atenolol, low-concentration samples are adsorbed to the PP tip
The sequence of initial results introduced in Part 3 involves the actual assessment of adsorption of low-concentration samples to PP tips. PP tips are easy to use during the preparation of low-concentration samples, but will increase the variability of analysis results. The TORAST-H Tip, in contrast, demonstrated adsorption suppression compared to the PP tip during preparation of low-concentration samples. Accordingly, in combination with TORAST-H Bio Vial, the contribution of this product to the reliability of LC/MS and other high-sensitivity analyses is highly anticipated.


