2-1) Increase in Solution Temperature
In situations such as when a solvent that is cold from being in storage is used in a warm room, the saturated solubility of gases gradually decreases as the temperature increases which results in excess dissolved gas forming bubbles. This is the same principle that causes carbon dioxide gas (CO2) to form more vigorously when soft drinks are warmed before opening.
Figure 2 shows the saturated solubility curves for O2 and N2 with respect to water, given a partial pressure of one atmosphere for the gases. Since actual air contains 20 % O2 and 78 % N2, if exposed to air, the amount of dissolved O2 and N2 is about 0.2 or 0.8 times the level indicated, respectively.
Note: 1 atmosphere (1 atm) = 1.013 × 105 Pa
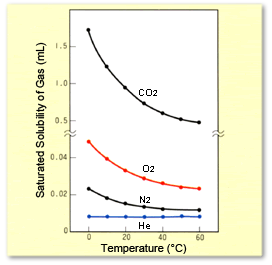
Effects of Temperature on Quantity of Dissolved CO2, O2, N2, and He (at partial pressures of 1 atm).








