Fluorescence Detection
When a compound is irradiated with light, electrons in the energetically stable ground state use the energy of the light to transition to energetically high and unstable excited states. Molecules that transition to the excited state lose energy due to heat or collisions with other molecules and return to the ground state. In this process, some compounds emit light with absorbed energy. This emission is called "photoluminescence" and includes fluorescence and phosphorescence. Fig. 1 shows the principle of fluorescence emission.
Absorbance detection is based on the absorption that occurs when a compound irradiated with light transitions from the ground state to the excited state. Fluorescence detection is a detection method that uses fluorescence emitted when a compound returns to the ground state after transitioning to the excited state.
Compounds containing functional groups with absorption bands can be detected by an absorbance detector, and only compounds with fluorescent properties can be detected by a fluorescence detector.
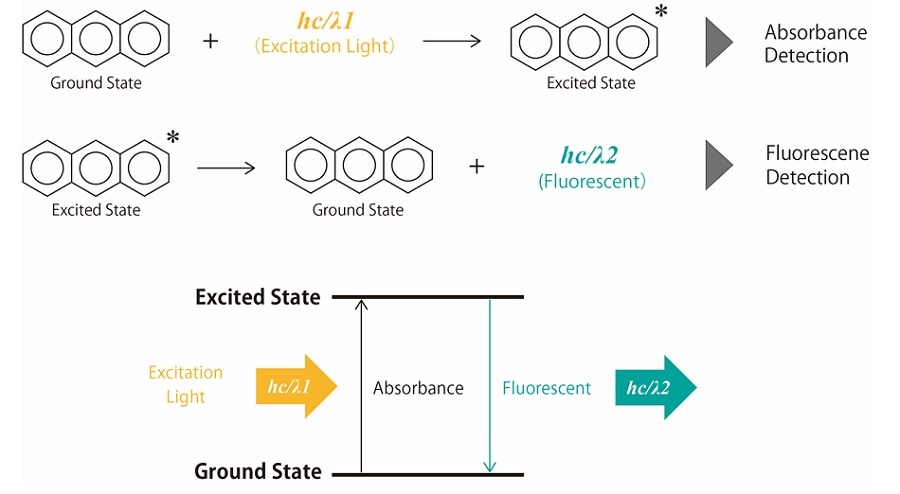
Fig.1 Principle of Fluorescence Emission
Fig.2 shows a schematic diagram of a fluorescence detector. A xenon lamp is generally used as a light source for the fluorescence detector. The light emitted from the lamp is separated into a light beam of a certain wavelength with the excitation grating, and then passes through the flow cell. When there is a substance in the flow cell that receives a specific wavelength of light and transitions to an excited state, it becomes a fluorescent compound. The light emitted from the compound is split by the emission grating and converted into an electrical signal according to its intensity by a photomultiplier.
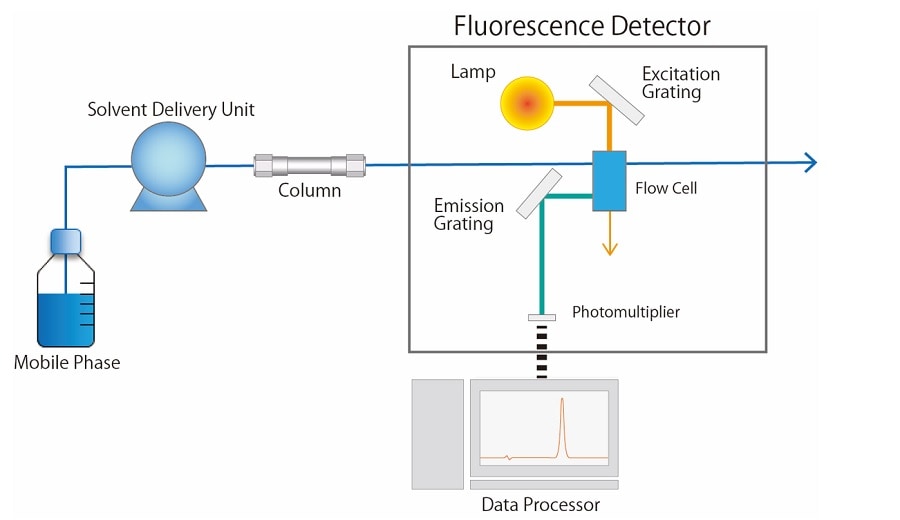
Fig.2 Schematic Diagram of a Fluorescence Detector
The advantage of a fluorescence detector is that it can be detected by excitation and fluorescence wavelengths specific to a particular substance, which leads to a higher selectivity of detection compared to other detectors. Furthermore, the analysis results would not be affected by noise derived from the mobile phase because the fluorescence detector is not capable of detecting any non-fluorescent compounds. This leads to direct detection of the light energy emitted by the fluorescence compounds, and also makes it possible to obtain higher sensitivity than absorbance detectors. Fig.3 shows an analysis example of isopropyl methylphenol (IPMP) , an active ingredient in medicated soaps using a fluorescence detector and an UV absorbance detector. IPMP can be detected by UV detector, however, the fluorescence detector is more useful for high sensitivity analysis. Table 1 shows the comparison of S/N ratio of IPMP (2 mg/L) calculated by the ASTM method for the fluorescence and the UV detector. An ASTM noise determination was done from the data point values from 15 minutes to 20 minutes at the interval of 0.5 minute in this analysis.
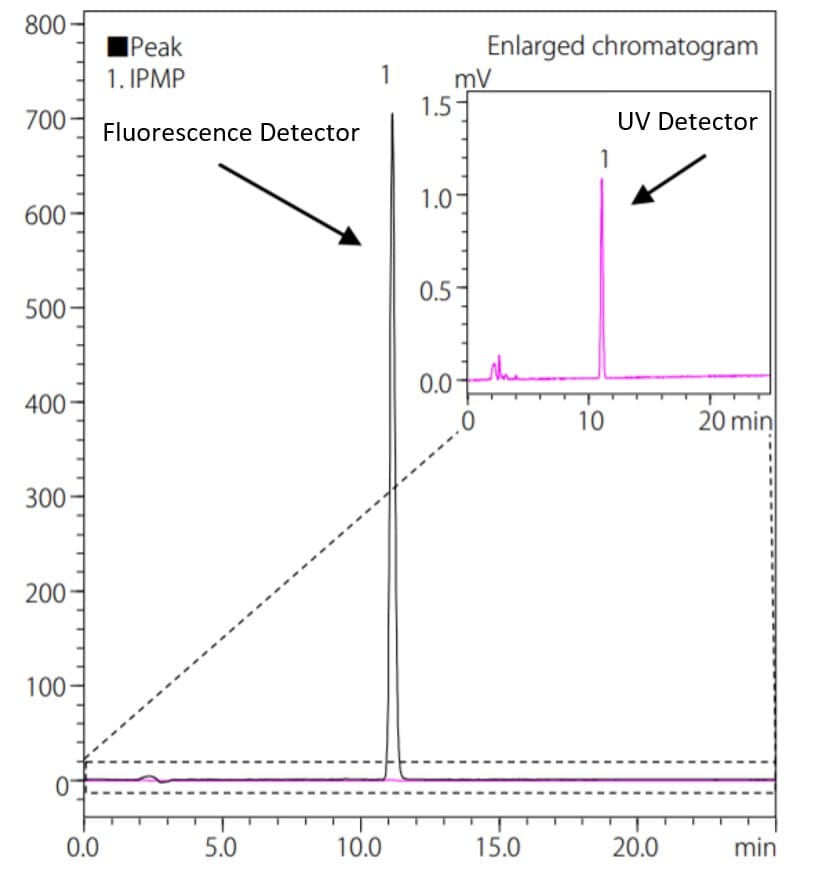 Fig. 3 Comparison of Chromatograms of IPMP
Fig. 3 Comparison of Chromatograms of IPMP
Standard Solution (2 mg/L)-
Table 1 Comparison of S/N Ratio of IPMP (2 mg/L)
Detection S/N Fluorescence 6289 UV 317
Reference:
In the case of this analysis, a 2 mg/L IPMP standard solution was analyzed. The concentration corresponds to the content of 0.1% that is equivalent to the lowest allowable content in real sample specified in the Japan’s Ministry of Health, Labour and Welfare (MHLW) assignment. In comparison with the absorbance detector, IPMP was detected with approximately 20 times higher sensitivity by the fluorescence detector.
Although only fluorescent compounds can be detected by a fluorescence detector, non-fluorescent compounds can be detected by using a derivatization method that can be converted into fluorescent compounds by reacting with various specific reagents.
Links:







