Changing the Carrier Gas
- Measures and Proposals to Reduce Helium Gas Consumption
- Reducing Consumption During or After Analysis
- Shutting off the Gas Supply After Analysis
- Changing the Carrier Gas
The following explains precautions and other information for switching the carrier gas from helium to nitrogen or hydrogen.
▼ Reviewing Analytical Conditions
Reviewing Analytical Conditions
When carrier gas is changed from helium to nitrogen or hydrogen, the optimum average linear velocity of the gas flowing in the column is different due to the difference in the gas properties, so it is necessary to consider analytical conditions to obtain the same result before and after the change. In particular, when nitrogen is used, the column efficiency varies greatly depending on the average linear velocity.
(In the figure below, HETP is a parameter representing column efficiency, and the smaller the parameter, the better the column efficiency.)
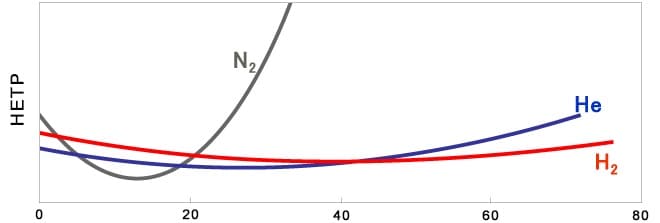
Average linear velocity (cm/sec)
Gas selector
It is recommended that you use a gas selector to consider the analysis conditions. The gas selector automatically switches the gas supplied to GC by connecting two types of gas. In the past, reconnecting pipes was required to analyze different types of gas. However, the gas selector is controlled by LabSolutions, and it can be automatically switched to continuous operation for each analysis.
EZGC™ Method Translator
EZGC Method Translator (Restek) can easily convert methods for helium to ones for hydrogen and nitrogen.
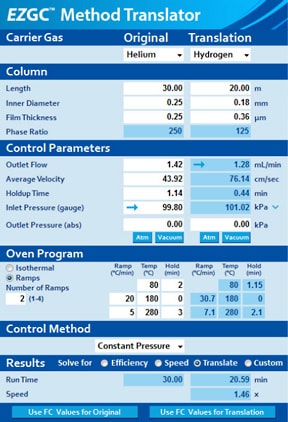
EZGC Method Translator
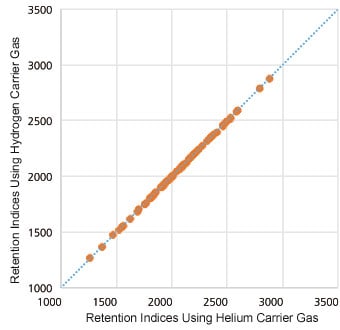
Correlation Between Retention Times with Hydrogen and Helium as the Carrier Gases for 70 Pesticides Translation to Analysis Conditions for Hydrogen as the Carrier Gas Utilizing EZGC Method Translator
Changing the Carrier Gas to Nitrogen
Carefully review the condition settings before switching the carrier gas to nitrogen.
Nitrogen gas (N2) is an inexpensive and safe gas. However, if nitrogen gas is used in a capillary GC system with the same analytical condition settings as for helium, separation capability may be reduced. If there are no adjacent peaks involved, then the carrier gas can be changed to nitrogen without changing the condition settings. If there are many adjacent component peaks and a certain level of separation is required, then the condition settings need to be reconsidered before using nitrogen gas.
Improving separation capabilities for nitrogen gas will require reconsidering the carrier gas linear velocity and temperature settings and will probably result in a longer analysis time. The vaporization state and detector sensitivity will probability change and the peak area percent might differ from when helium gas was used.
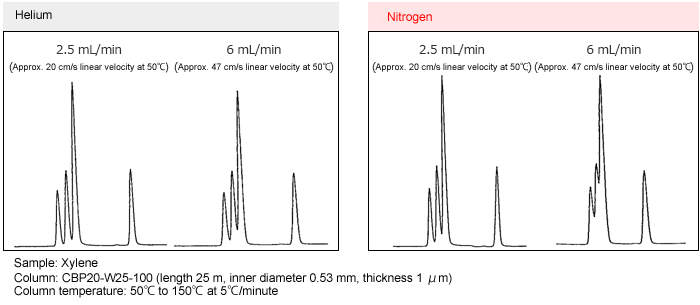
Separation with Helium Gas and Nitrogen Gas
Separation examples for helium and nitrogen gases at different linear velocities are shown below.
Using helium gas, the separation is virtually unchanged across the range from 20 to 47 cm/s linear velocity. With nitrogen gas, however, the separation deteriorates at 47 cm/s linear velocity. This occurs because the optimal linear velocity for separation is lower with nitrogen than with helium and the optimal linear velocity range for nitrogen is narrower than for helium. Also, in the case of temperature rise analysis, the linear velocity changes during temperature rise depending on the gas control mode. However, in the case of Shimazu GC, by using constant linear velocity control, optimum separation can be obtained using nitrogen gas.
Changing the Carrier Gas to Hydrogen
In an increasing number of cases, hydrogen gas is being considered as a carrier gas for use in GC analysis. Hydrogen (H2) gas is easier to obtain and less expensive than helium gas and offers higher separation performance for a given linear velocity. Therefore, if it is used safely, it can significantly reduce running costs. On the other hand, hydrogen is a dangerous gas that can easily explode. The following explains the risks, precautions, and other information about using hydrogen gas safely.
[Caution]
- Be sure to use hydrogen gas safely, in accordance with manuals relevant to using respective devices safely. Also, read the hydrogen gas precautions included in the gas chromatograph instruction manual.
- Changing the type of carrier gas used may require reviewing the specified analytical conditions.
- Because GC pretreatment systems also use other gases besides carrier gases, it might not be possible to use hydrogen as the carrier gas with some instruments.
| If a leak error occurs using an electronic flow controller and APC/AFC unit, even though the gas supply pressure is normal, stop using the system and request service from a Shimadzu representative. |
| For gas chromatographs with a manually specified hydrogen gas flowrate, if the flowrate or pressure is much higher (or lower) than normal, then check for gas leaks, including from the pressure control valve. If no leaks are found, leakage does not stop, or normal performance is not restored after stopping the leak, stop using the system and request service from a Shimadzu representative. |


