July 3, 2024 | News & Notices
Contributing to the Rapid Development of Pharmaceuticals by Automating Pretreatment in Antibody Glycan Analysis
Release of the MUP-3100 Fully Automated Sample Preparation Module for Glycan Analysis

Product Photo: MUP-3100 Fully Automated Sample Preparation Module for Glycan Analysis
Shimadzu Corporation has released the MUP-3100 fully automated sample preparation module for glycan analysis. This product automates the time-consuming pretreatment process in the analysis of antibody glycans, which is required for the research and development and quality control of antibody pharmaceuticals. A 6-axis robot provides labor savings and increases efficiency with respect to processes that were conventionally performed manually. This enables not only continuous operations, but also eliminates procedural mistakes, ensures the safety of operators, and lessens the burden on them. It is expected to be used by pharmaceutical companies as well as contract manufacturing organizations (CMO) and contract development and manufacturing organizations (CDMO), where it will contribute to speeding up the research and development of pharmaceuticals by heightening the safety and efficiency of operations. This instrument is exclusively for antibody glycan analysis using the Auto-EZGlyco™ mAb-N Kit for SHIMADZU from Sumitomo Bakelite Co., Ltd.
The process known as pretreatment is required for instrumental analysis of samples using liquid chromatographs (LC) and liquid chromatograph mass spectrometers (LC-MS). It is troublesome because it uses multiple reagents, a centrifuge, pipettors, and other instruments and devices. Additionally, operations such as dispensing, which involves weighing out an amount of a reagent, and the addition of reagents require skill and experience. In research and development and quality control of pharmaceuticals, troublesome pretreatment processes are a burden on researchers and engineers, and there is a strong demand for automation.
Antibody pharmaceuticals consist of proteins (antibodies) produced in cells using genetic modification technology. They are expected to provide high therapeutic value and fewer side effects because they target antigen landmarks on the surface of cancer cells. At the same time, glycans (compounds consisting of sugar chains) linked to the surfaces of antibodies make production control difficult because of their impact on the effectiveness and safety of antibody pharmaceuticals, so glycan components must be analyzed using LC or LC-MS. In the development of biosimilars which are follow-on antibody pharmaceuticals, comparative testing of glycans with prior products becomes a quality control criterion. This product automates pretreatment in antibody glycan analysis, which lessens the burden on operators, and helps provide high analysis accuracy and repeatability based on the correct sequence of procedures.
In addition to this product, Shimadzu provides a wide range of products that are useful in the development of antibody pharmaceuticals and biosimilars. These include ICP mass spectrometers to analyze the metallic elements related to glycan synthesis, as well as LC-MS software to analyze the substrate sugars of glycans. With these products and technologies, Shimadzu is expanding sales in North America, the main biopharmaceutical market, the biosimilar industry, and the Asian region, where CMO and CDMO growth has been striking. At the same time, by improving the operational efficiency of pharmaceutical companies, CMO, and CDMO, we are contributing to speeding up the research and development of antibody pharmaceuticals.
Features
1. Accurate Repeatability of Pretreatment Processes by a 6-Axis Robot
A 6-axis robot performs a preregistered pretreatment sequence including dispensing using pipettors and the conveyance of samples to a centrifuge. While faithfully reproducing the conventionally manual operations, pretreatment is performed quickly and reliably, without burdening the operator, which helps to provide stable analytical results. Using the Sumitomo Bakelite pretreatment kit with this instrument, treatment times can be shortened from approximately 2 days to 6 hours (for 24 samples), enabling the treatment of up to 48 samples per day.
2. Automatic Check Function Supports Stable Operation
A camera within the instrument automatically checks the positioning and amount of reagents and consumables before operations start, preventing treatment malfunctions and stoppages due to positioning mistakes and other human errors. In addition, during automatic operation, the system checks the attachment/detachment of pipette tips as well as the conveyance of the samples. The objective is to complete treatment even if something unforeseen occurs by using the retry function. A camera with a video function is also installed so that downtime when a problem occurs can be decreased using functions that automatically extract and save video clips back through the time line, and store the operating logs.
3. Functions for Enhanced Regulatory Compliance
User management software (optionally available) is used to set rights for each account, which prevents unintentional changes to the analysis procedures. Furthermore, pretreatment result reports are automatically created and stored, which improves traceability (the ability to trace the analysis history). In addition, the instrument is equipped with routine inspection support functions that can easily check the pipettor dispensing accuracy before starting, which heightens the reliability of the analysis results.

Product Photo: MUP-3100 in Use
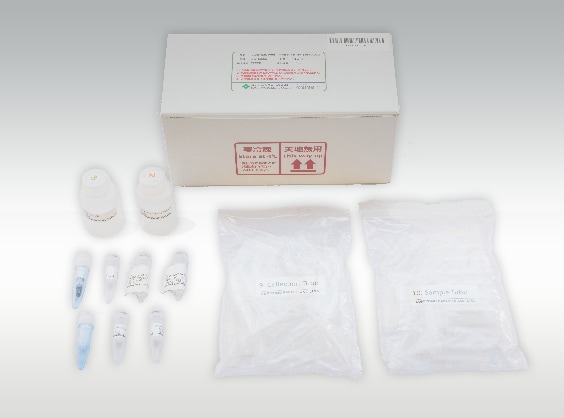
Reference Photo: Auto-EZGlyco™ mAb-N Kit for SHIMADZU Antibody Glycan Analysis Pretreatment Kit (Manufactured by Sumitomo Bakelite Co., Ltd.)
For more details, visit
MUP-3100


