Vacuum Section of ICP-MS System Components
Basics of Inductively Coupled Plasma Mass Spectrometry
1. Interference Removal, Mass Separation, and Detection
Ions drawn into the vacuum from the interface are then carried into a region with high vacuum (<0.1 Pa) before being introduced to a collision/reaction cell. While passing through the cell, ions collide and/or react with gas occupying the cell (He, H2, etc.). These collisions and reactions remove interfering components that would otherwise be detected at the same mass to charge ratio (m/z) as the analyte of interest. The analyte elements are then separated by a quadrupole mass spectrometer based on m/z.

2. Collision/Reaction Cell
In ICP-MS analysis, the following types of ions may share the same m/z as analyte ions and appear as spectral interference.
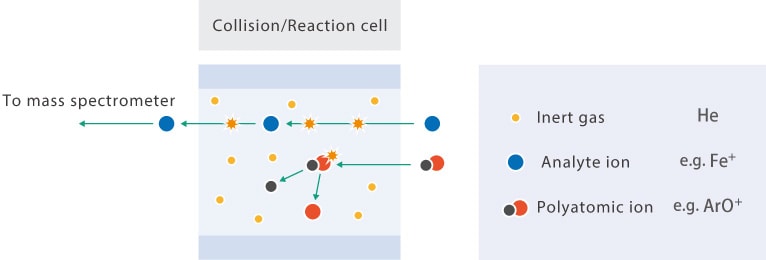
- Polyatomic ions: Quazi-stable combinations of ions attracted to one another within the plasma. While these can be nearly any combination of ions (oxides, nitrides, carbides) the most common recombination is between the Ar used as plasma gas and matrix elements from the sample (e.g. analyte ion: 56Fe+, interfering ion: 40Ar16O+)
- Doubly charged ions: Ideally we seek to remove a single electron during ionization, however some ions lose a second electron and become doubly charged. These ions appear at an m/z that is half the mass of the element ion (because m/(z*2); e.g., 78Se+, interfering ion: 156Gd2+)
- Isobaric ions: Ions with equal masses but different numbers of protons and neutrons (e.g., 114Cd+, interfering ion: 114Sn+)
Whenever possible avoid this interference by measuring a different mass number. When this is not possible, the collision/reaction cell is used to collide and react interfering ions and/or polyatomic clusters with specific gases, thereby reducing and eliminating isobaric spectral interference. An octopole ion guide is used to refocus analyte ions that stray off course due to these collisions.
The different features of collision/reaction cells can be summarized as follows. Collision-based interference removal uses inert He gas and can be used with almost all analyte elements because the collisions create no by-products. Meanwhile, reaction-based interference removal separates analyte ions from interfering ions based on their reactivity with a reaction gas. This approach provides more powerful interference removal but is only effective for specific elements.
| Collision-based | Reaction-based | |
|---|---|---|
| Gas used | Inert gas He |
Reactive gas H2, NH3, O2, etc. |
| Suitable analyte elements | Almost all elements except for low mass elements | Se, Fe, etc. (with H2 gas) |
| Interferences removed | Polyatomic ions | Polyatomic ions, doubly charged ions, isobaric ions |
| Advantages | No special knowledge required as no by-products are produced Multiple elements can be analyzed simultaneously with a single set of conditions |
Highly effective interference removal for specific elements Can also effectively remove doubly charged ion and isobaric ion interferences |
| Disadvantages | Because analyte elements also collide with the He gas, the signal intensity of analyte ions is reduced compared to no-cell gas Cannot remove doubly charged ion and isobaric ion interferences (can be corrected for using inter-element correction and interference correction equations) |
Because removal is based on reactivity, the reactivity of each element must be known and conditions must be configured for each element Cannot analyze multiple elements simultaneously with a single set of conditions Generates by-products that may create more interference Installation environment requires special consideration due to use of reactive gas |
Collision-Based Removal of Interferences (1): Collision-Induced Dissociation (CID)
Collisions between polyatomic atoms and He gas in the cell remove interference by splitting the polyatomic ions. This method of removing interference by causing the dissociation of polyatomic ions is called collision-induced dissociation.
Collision-Based Removal of Interferences (2): Kinetic Energy Discrimination (KED)
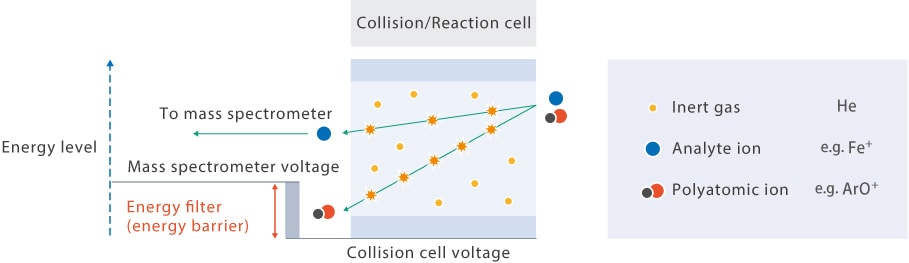
Polyatomic ions have a larger collision cross-section area than monoatomic ions and thus collide more frequently with He gas. This results in a greater loss of energy from polyatomic ions.
An energy filter (barrier) is placed between the collision/reaction cell and the mass spectrometer. Polyatomic ions with lower kinetic energy are unable to pass through this barrier and are prevented from reaching the mass spectrometer. This kinetic energy-based method of separating polyatomic ions from analyte ions is called kinetic energy discrimination.
Reaction-Based Removal of Interferences
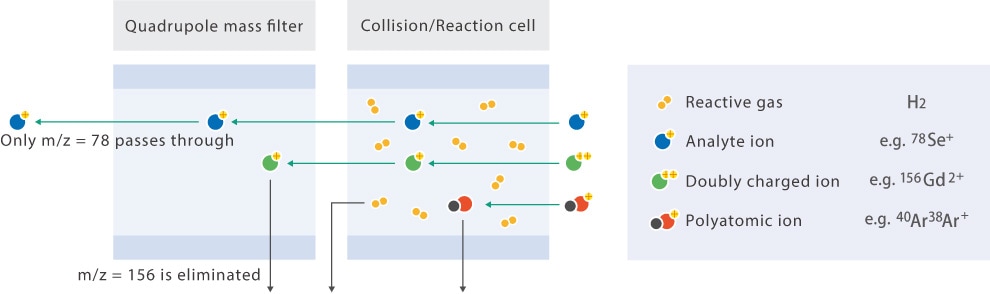
A highly reactive gas is used to remove interferences based on the different chemical reactivity of each element. H2 is the most commonly used reaction gas. An example interference removed by H2 is 40Ar38Ar interference on 78Se. The reaction between Se and H2 is endothermic and thus occurs very rarely, while the reaction between Ar and H2 is exothermic, occurs more readily, and removes interference as shown below.
Ar2+ + H2 → ArH+ + Ar + H (hydrogen atom transfer reaction)
ArH+ + H2 → Ar + H3+ (proton transfer reaction)
3. Quadrupole Mass Filter
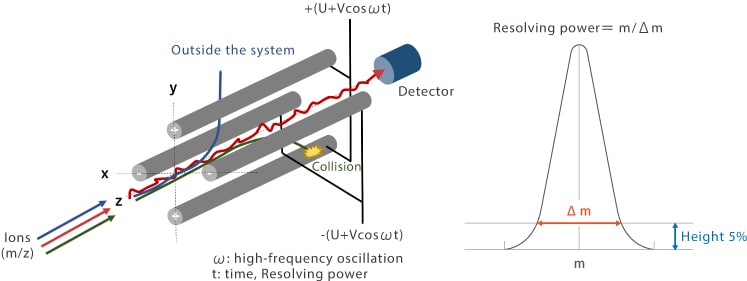
A quadrupole consists of four cylindrical metal rods arranged in parallel. Voltage of the same polarity is applied to each opposing pair of rods (or electrodes) in the quadrupole. An electric field of rapidly varying phase is generated within the quadrupole by applying a combination of a direct current voltage U and a high-frequency alternating current voltage (V cos ωt). The DC voltage (U) and high-frequency alternating current voltage (V) are adjusted to guide ions of interest into the detector.
Ions enter the quadrupole region along the central axis (z-axis) and the electric field causes ions passing through the quadrupole to oscillate in x and y directions. Under a given set of conditions, ions of a specific m/z experience "stable oscillation" in the electric field, allowing them to pass through the quadrupole and reach the detector. Ions with other m/z experience unstable oscillation and collide with an electrode or fly out of the quadrupole region and are not detected. Resolving power is a measure of a system’s ability to achieve spectral separation and is represented by m/Δm, where Δm is the peak width at 5 % the peak height at a given mass (m). In quadrupole mass spectrometers that have a quadrupole mass filter, Δm is constant and independent of mass and resolving power is proportional to mass. Although elements are typically separated by a mass difference of around 1 amu (atomic mass unit), analysis is performed based on an Δm of around 0.65 to 0.8 amu.
4. Detector
A secondary electron multiplier detector converts ions exiting the mass filter into readable electrical signals. The electron multiplier is comprised of many dynodes where a negative high voltage is applied to the first dynode at the entrance. When an ion collides with the first dynode, it causes the emission of multiple secondary electrons. These electrons are accelerated by a difference in applied voltage and, in doing so, collide with the nearest inner wall, causing an emission of more secondary electrons. This process of colliding and emitting secondary electrons occurs repeatedly, amplifying the number of electrons 106 to 108 times before the electrons enter the detection circuit.
Signals are detected by pulse detection or analog detection. Pulse detection converts the electron amplified 108 times into a voltage pulse and counts the number of voltage pulses (ions) per unit time. Analog detection takes the current amplified 104 to 106 times, converts this current to a DC voltage in the detection circuit and is subsequently measured. Detection automatically switches from pulse to analog when the ion intensity saturates the pulse detection method. Ion intensity measurements are represented in terms of cps (counts per second) and defined as the number of ion counts measured by the detector per second.

Analog detection: After the current is amplified 104 to 106 times, the current is converted to a DC voltage by the detection circuit and the DC voltage is measured over a unit of time.






