Oligonucleotide Therapeutics - Separation and Analysis
The chemical synthesis and purification processes used to manufacture oligonucleotide therapeutics can generate many impurities, such as defective nucleotide elongation or defective removal of protective groups. This requires that the target nucleic acids must be properly separated and purified.
Related Products
LCMS-2050
Ultra High Performance Liquid Chromatograph
The Nexera XS inert uses proprietary technology to minimize exposure to metal ions on the internal surfaces of the system while still providing all the same features as other Nexera series models. By eliminating adsorption caused by interactions between nucleic acid molecules and metal ions, the system can prevent peak tailing and avoid decreases in recovery rates for target components As a result, data with good peak shapes can be obtained with high sensitivity, even for low-concentration samples containing trace quantities of nucleic acids.
LabSolutions™ MD
Automatic Optimization of Gradient Conditions with AI Algorithm
LabSolutions MD enables the development of efficient analysis methods based on the “Analytical Quality by Design” (AQbD) approach, in which analysis methods are developed based on scientific evidence and risk. It can complete a series of workflow steps, from creating an analysis schedule based on the “design of experiments” method, automating the analysis by switching the mobile phase, column, and LC parameters, determining optimal analysis conditions by constructing a design space, to evaluating robustness.
Nexera Prep
Preparative Purification Liquid Chromatograph
Nexera Prep offers solutions to the challenges of preparative purification, such as simplifying and scaling up preparative workflows and providing a simulation function that supports fraction parameter optimization. The LH-40 liquid handler is equipped with auto-injection and fraction collection functions that enable flexible system configuration based on your application.
Shim-pack Scepter Claris
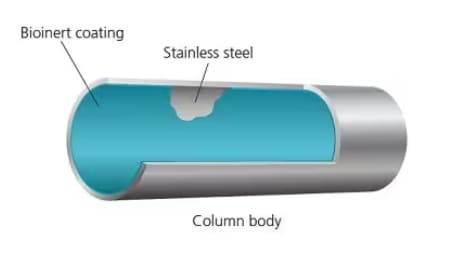
HPLC Column
The body of the bio-inert HPLC column is coated with a newly developed bioinert coating, and the column is packed with an organic silica hybrid stationary phase. Highly reproducible, high-sensitivity nucleic acid analysis results are obtained from the first injection. Another feature is high durability.